Provider of Authorized Medical Device License Keys & OEM-Compliant Option Activation
Looking for dependable, audit-ready ways to unlock features on your medical systems? We provide:
Fast, secure, device-locked license keys for leading brands: Siemens, GE HealthCare, Philips, Canon, Samsung, Mindray.
Option enablement across modalities—Ultrasound, CT, MRI, C-Arm—including HDlive, STIC, CW/Color Doppler, Elastography, CT Perfusion, Iterative/AI Recon, MRI Cardiac, DSA/Roadmap/3D Rotational.
Encrypted delivery, medical device license OEM-compliant activation, and audit documentation for warranty and compliance teams.
Tailored bundles matched to your serial/build and clinical workflow—often live the same day.
📩 For details and WhatsApp-only pricing, contact us:
WhatsApp: +967775455559 • Click-to-Chat: https://wa.me/message/NRDFVKNYDTUNM1
Let us help you safely maximize the capabilities of your imaging equipment—quickly, compliantly, and with professional support. 🌟
Table of Contents
ToggleIntroduction to medical device license
Buy medical device license safely and legally—without guesswork or risk. In this guide, we clarify the difference between software medical device license and regulatory approvals (FDA/EU MDR), then show how to unlock features on ultrasound, CT, and MRI systems with medical device license OEM-compliant activation. You’ll learn the secure process (system info → compatibility check → encrypted, device-locked key → install & verify), pricing drivers and ROI, plus how to protect warranty, compliance, and cybersecurity while avoiding common pitfalls. Ready to activate the right options and elevate care? Let’s dive in.

Medical Device “License” Explained (Software vs. Regulatory)
Overview
When people say “medical device license,” they often mean two very different things. One is a software license/option key that unlocks features already present on your device. The other is regulatory licensing/approval (e.g., FDA, EU MDR) that governs market authorization and compliance. This section separates the two, shows where each applies, and explains why the distinction matters for purchasing, service, and risk management.
A) Software License / Option Key
A software license (also called an option key or service key) is a machine-locked code that enables specific features on modalities like ultrasound, CT, or MRI. Keys are generated from device identifiers (serial, build, license report) and installed via the system’s license manager. Typical options include 4D/HDlive, CW/Color Doppler, Elastography, and Cardiac/Perfusion packages. The key doesn’t change hardware; it activates existing capabilities securely with a documented audit trail.
In practice, an OB/GYN clinic with a GE ultrasound might add HDlive and STIC to expand prenatal services, or a CT site could enable dose-reduction reconstruction to improve image quality without replacing scanners. After an HDD swap, a refurbisher may restore lost options using documented keys. Properly issued keys are encrypted and device-specific, minimizing cybersecurity and warranty risks while delivering fast ROI versus buying new equipment.
B) Regulatory Licenses & Approvals
Regulatory licenses and approvals are market authorization frameworks, not feature unlocks. Examples include FDA pathways (e.g., 510(k) in the U.S.), EU MDR CE marking in the European Union, MHRA/UKCA in the U.K., and SFDA in Saudi Arabia. These apply to manufacturers and distributors (and sometimes facilities), ensuring safety, quality systems, and intended use claims—distinct from end users enabling software options on approved devices.
Turning on a software option does not grant regulatory clearance or change intended use. Hospitals must still meet institutional, national, and payer requirements. A radiology center activating perfusion analysis on an approved CT must ensure the indication is cleared for that model/firmware in its market. Always align with the OEM EULA, local regulations, and institutional policies; when in doubt, consult compliance or the manufacturer.
C) PAA: “What is a medical device license?”
In purchasing conversations, “medical device license” usually means a software option key that unlocks premium features—Doppler, 4D, Elastography, Cardiac—on equipment you already own. It is purchased, delivered, and installed digitally, then verified in the system menu. Think of it as upgrading from a base to a feature-complete configuration without hardware changes, downtime, or rescheduling patients.
In regulatory contexts, the same phrase refers to market approvals (FDA/EU MDR/MHRA/SFDA). That use case concerns who can sell or market devices and under what conditions—separate from enabling features on an installed system. This guide focuses on the software-license meaning: how to buy safely, verify compatibility, install correctly, and preserve warranty, cybersecurity, and audit documentation.
D) Why the distinction matters
Confusing “software license” with “regulatory license” can cause expensive mistakes. Buying a cheap generic keygen risks firmware corruption, malware, and warranty loss, while assuming a software key replaces regulatory obligations can trigger compliance findings. Reputable activation follows OEM-compliant workflows: device-locked encryption, documented chain of custody, license logs, and post-install verification suitable for audits and biomedical QA.
Operationally, clarity saves time and money. If your goal is more capability (HDlive, perfusion, elastography), you need a software option key matched to your model, firmware, and probes. If your goal is market access or institutional approval, you need regulatory pathways. Use a pre-purchase checklist: confirm device identifiers, export the license report, verify indications and compatibility, and plan a stable power window for installation and testing.
Who Should Buy a Medical Device License?
Overview
Not every site needs new hardware to expand capabilities. Buying a medical device software license makes sense when you want to unlock approved features on equipment you already own, restore lost options after service events, or standardize capabilities across multiple rooms and sites. The right license delivers faster ROI, less downtime, and a cleaner compliance trail than replacing devices.
A) Audiences: Who benefits most?
Clinical owners and administrators gain predictable upgrades without capital replacement. Adding options such as HDlive, CW/Color Doppler, elastography, cardiac or perfusion packages lets a service line launch new exams quickly, price them confidently, and improve scheduling utilization. For multi-site groups, licenses help harmonize protocols so technicians can move between rooms without re-learning different feature sets.
Biomedical/HTM teams value licenses because they are device-locked and auditable. After hard-drive swaps, software reloads, or mainboard replacements, teams can re-apply the licensed options with minimal disruption. Refurbishers and authorized resellers also rely on proper licensing to deliver feature-complete systems, document chain-of-custody, and pass incoming QA. Distributors use licenses to tailor configurations for tenders without over-spec’ing hardware.
B) Use cases: When to buy a license?
Feature expansion on installed systems. An OB/GYN clinic with a GE ultrasound may add STIC and HDlive to promote advanced prenatal imaging; a CT department can enable dose-reduction reconstruction or perfusion for stroke pathways. These upgrades typically go live after a short maintenance window, avoiding the lengthy procurement, siting, and physics acceptance of new equipment.
License restoration and standardization. After an HDD failure or OS rebuild, previously enabled options can disappear. Purchasing the correct license set restores the original capability and maintains warranty/compliance records. Networks often buy identical license bundles for Ultrasound, CT, and MRI rooms to standardize workflows—for example, enabling CW Doppler across all echo rooms or matching cardiac packages across MRI bays, which simplifies training, service, and protocol governance.
OEM vs. Trusted Provider: How to Buy Safely
Overview
Choosing where to buy a medical device software license is as important as the license itself. You’ll typically pick between the OEM (manufacturer) route and a trusted third-party specialist. Each path carries trade-offs in cost, turnaround time, documentation, and warranty alignment. This section explains both routes, clarifies the legal/compliance landscape, and gives you a practical due-diligence framework to avoid costly mistakes.
A) The OEM Route (Manufacturer Path)
Buying from the OEM—such as GE HealthCare, Philips, Siemens Healthineers, Canon Medical, Samsung Medison, Mindray—offers the most straightforward warranty alignment and change control. Licenses are recorded inside the manufacturer’s systems, training material is consistent, and upgrades may bundle with broader service plans. For hospitals with strict governance, this route can simplify audits because procurement, activation, and documentation live within a single vendor ecosystem.
The trade-offs are typically higher prices and longer lead times. Some options require contract addenda, site IDs, or scheduled engineer visits. A cardiac service line that needed CT perfusion, for example, faced a four-week window for OEM scheduling and higher per-option fees; they chose it anyway because their internal policy prioritized full manufacturer chain-of-custody and centralized support.
B) The Trusted Specialist Route (Verified Third-Party)
A reputable specialist focuses on multi-OEM licensing and can move quickly with encrypted, device-locked keys, compatibility checks, and remote guidance. Clinics often save 30–60% and go live faster, which matters when a new service (e.g., OB 4D or CT dose-reduction) is the bottleneck to revenue. Good providers issue machine-specific files, activation reports, and post-install verification so your biomedical team has an auditable trail.
Quality control is everything. Insist on documented workflows (collect system info → validate build/probes → issue encrypted key → verify options), refund/replace policies for incompatibility, and secure delivery (checksum or digital signature). A women’s health center that enabled HDlive + STIC via a specialist went live the same day; the provider’s activation log and invoice satisfied the hospital’s QA review and internal audit committee.
C) Legality & Compliance (PAA: “Is it legal to buy medical device license ?”)
Legality hinges on how the key is generated and whether it respects the OEM EULA and local law. It is legitimate to purchase authorized, device-specific keys that enable options already cleared for your model/firmware in your market. What is not acceptable is using cracks, generic keygens, or files that bypass protections; those can violate IP law, void warranties, and create cybersecurity exposure.
From a compliance standpoint, keep a paper trail: purchase order, serial/build identifiers, feature list, activation report, and who performed the change. Use secure channels for file transfer (TLS links, VPN, or encrypted email) and store hashes/signatures with the license file. During accreditation or ISO audits, this evidence demonstrates proper change management and reduces risk findings for unauthorized modifications.
D) Due-Diligence Framework (How to evaluate a seller)
Start by validating identity and track record: years in operation, healthcare references, and examples across multiple OEMs. Ask for a sample activation report (with PHI removed) to see what proof you’ll receive. Confirm device-locked encryption, build compatibility, and post-activation verification. A solid quote itemizes option names, part codes (if applicable), turnaround time, support window, and refund/replace terms.
Compare quotes on total value, not price alone. Consider whether the vendor: (1) checks probe/firmware compatibility, (2) provides remote screen-share during install, (3) issues audit-ready documents, and (4) offers escalation paths. One radiology network standardized MRI cardiac packages via a specialist because the provider promised 24-hour replacement keys and kept an internal map of each scanner’s build history—saving days during future OS upgrades.
E) Red Flags & Risk Mitigation
Be wary of sellers who refuse to tie a license to your specific serial/build, provide only generic files, or pressure you to disable antivirus/Windows protections. Vague timelines, no refund policy, and reluctance to share sample documentation are common signals of risk. If a quote is dramatically cheaper than market norms without explaining how compliance is preserved, assume corners are being cut.
Mitigate risk by piloting a single system before bulk purchases, performing a full backup, and scheduling activation during a stable power window with biomedical oversight. Verify features immediately in the options menu and run a test scan relevant to the unlocked capability. Document everything—who, when, what options—so your facility can pass internal QA, external audits, and warranty inquiries with confidence.
Supported Modalities & Popular Options
Overview
Software licenses unlock capabilities that already exist within your system, turning base configurations into clinically capable platforms without replacing hardware. The right options vary by modality and service line. Below, you’ll see how ultrasound, CT/MRI, and C-arm/angio systems commonly use medical device license to enable premium features—what those features do, when they make sense, and what to verify before purchase to keep workflows compliant and efficient.
A) Ultrasound (GE Voluson/Vivid/LOGIQ; Philips EPIQ/Affiniti; Siemens ACUSON; Samsung HS; Mindray Resona/DC)
Ultrasound licenses typically enable 4D/HDlive, STIC, SonoAVC, CW/Color Doppler, strain/elastography, and specialty packages (cardiac, vascular, OB). On GE Voluson and Vivid, for example, HDlive and CW Doppler move a system from general imaging to advanced OB/GYN or echo. On Philips EPIQ/Affiniti, elastography and TrueVue-style rendering sharpen soft-tissue assessment and patient communication. Keys are device-locked, so compatibility with firmware and probe families must be confirmed.
A practical scenario: a women’s health clinic activates HDlive + STIC to expand prenatal services without buying a new console, while an echo lab enables CW Doppler to perform quantitative valvular assessments. After a hard-drive replacement, a refurbisher restores lost options using documented license reports. Post-activation, sonographers validate presets and run a short QC session to confirm Doppler angles, frame rates, and 4D rendering quality match protocol expectations.
B) MRI & CT
MRI licenses often cover cardiac packages, perfusion, spectroscopy, diffusion, parallel imaging, and advanced post-processing. These unlock gated acquisitions, myocardial tagging, or quantitative maps that would otherwise require referral. CT licenses commonly add iterative/AI reconstruction, cardiac gating, perfusion, dual-energy decomposition, and dose-reduction toolsets. Because these are algorithmic features, activation usually means new menus and reconstruction kernels rather than hardware swaps.
Consider a stroke pathway: enabling CT perfusion allows faster decision-making for thrombectomy candidates, integrating seamlessly with existing scanners once the option is licensed and validated. A cardiac service may license MRI cardiac to run stress perfusion or viability studies on a schedule that fits their patient flow. Teams plan an acceptance window for physics/QC, confirm the software build supports the options, and update protocols so technologists see the new series and reconstruction presets immediately.
C) C-Arm & Angio
Interventional suites rely on licenses for DSA (digital subtraction angiography), Roadmap overlays, 3D rotational angiography, advanced fluoroscopy processing, and dose-monitoring features. These options improve vessel visualization, guide catheter navigation, and support complex endovascular work without a full system replacement. Because image chains are tightly integrated, ensure the license is tied to the generator/detector and that any calibration steps are scheduled after activation.
An endovascular team might add DSA + Roadmap to a base system to improve peripheral interventions, while an orthopedic OR enables 3D rotational modes for complex trauma and pedicle screw verification. A short training huddle helps surgeons and scrub staff adopt overlay controls and storage workflows. Biomedical engineers verify dose reporting and post-activation logs, documenting who enabled what and confirming the features appear in the angio protocol library for governance and audit.
Step-by-Step: How to Buy & Activate (Securely)
Overview
This section walks you through a safe, repeatable workflow to buy and install a medical device software license. You’ll collect precise system identifiers, validate firmware/probe compatibility, purchase an encrypted, device-locked key, and complete installation via the license manager with post-activation verification. Following these steps reduces downtime, preserves warranty and compliance, and prevents common errors that cause invalid keys, failed activations, or audit issues.
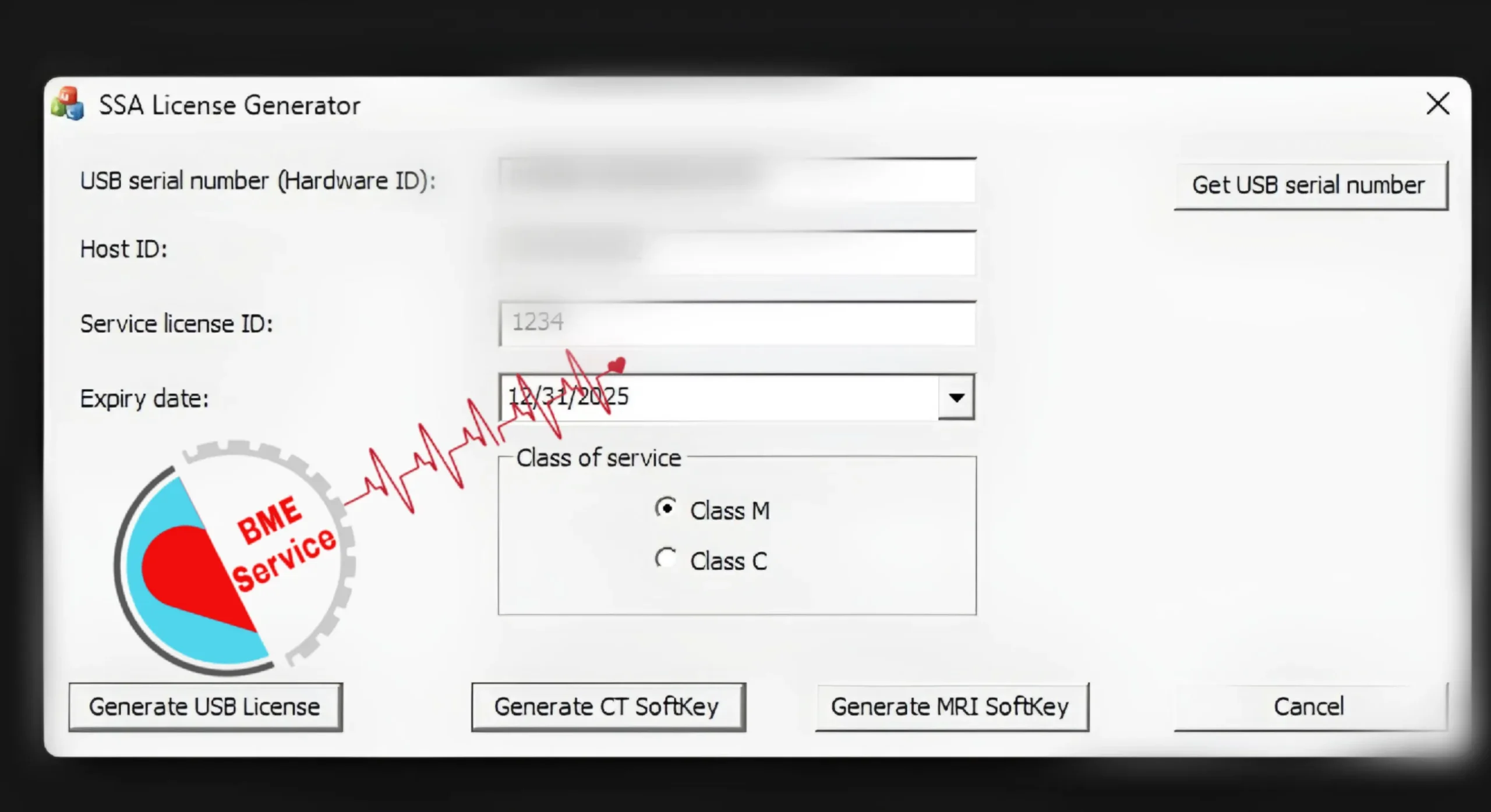
A) Collect system information (serial, software build, license report)
Start by gathering exact identifiers: model, serial number, software/build version (e.g., GE BTxx, Philips EPIQ x.x.x), and the license/options report exported from the service menu. Capture the probe list, storage type (HDD/SSD), and any recent hardware changes. Save screenshots/PDFs to a shared folder with timestamps. These artifacts are the “fingerprint” the provider uses to generate a device-locked key.
Accuracy matters. A refurbisher once mistyped a single digit in the build ID and received a key that wouldn’t load; correcting the ID resolved it immediately. Create a small “intake” checklist: verify serial against the rear label, export the license report twice, and include a short video of the About/Service screen. This extra five minutes typically saves hours of back-and-forth later.
B) Run a compatibility check (probes/options vs. firmware)
Match intended options to your firmware and hardware. Some capabilities (e.g., CW Doppler, HDlive/4D, CT perfusion) require minimum software levels, specific probe families, or GPU/CPU features. Ask the seller to confirm with an option–build matrix and to flag any prerequisites such as driver packs or minor software updates. If your system is end-of-support, plan for an OEM-approved upgrade before licensing.
A practical example: an echo lab wanted CW Doppler on a mid-generation console. The probes supported CW, but the installed BT level did not. A minor software update brought the system into spec and the license then applied cleanly. Similarly, a site enabling CT perfusion confirmed the scanner had at least 64 slices and adequate reconstruction performance, avoiding a costly license that their hardware couldn’t exploit.
C) Purchase & receive an encrypted, device-locked key
Submit your identifiers and receive a quote that itemizes option names, delivery time, and refund/replace terms for incompatibility. Reputable providers deliver a machine-specific file plus a hash/checksum or digital signature. Expect secure delivery (TLS link, encrypted email, or portal) and documentation: invoice, activation instructions, and a template for your internal change log. Avoid generic files or anything that requires disabling antivirus.
A women’s health center licensed HDlive + STIC through a trusted specialist and went live the same day. When a mainboard replacement occurred months later, the provider re-issued a replacement key under the contract at no cost. Savings versus OEM pricing were ~40%, but more importantly, they had a clean paper trail—PO, license report, activation log—that satisfied both biomedical QA and the hospital’s audit committee.
D) Install via license manager; reboot; verify
Schedule a short maintenance window with stable power (preferably on UPS). Back up presets and export the current license state. Load the key through the system’s license manager, following the vendor’s sequence (close exams, stop services, apply key). Some platforms require a reboot to bind the license. Record who performed the change, exact time, and any on-screen confirmations or error codes.
Verification is non-negotiable. Open the options/features menu and confirm the new capabilities appear as Enabled/Active. Run a focused QC: for ultrasound, validate Doppler angles and frame rates; for CT, test the new reconstruction kernel on a phantom; for MRI, confirm gated sequences and post-processing workflows. Export a fresh license report, attach it to your asset record, and note any protocol updates pushed to technologists.
E) PAA — “How long does activation take?”
Typical timelines range from same-day (trusted specialist with complete identifiers) to several weeks (OEM scheduling, contract addenda, or on-site engineer). Most ultrasound options activate within 1–3 hours of maintenance time; CT/MRI post-processing may require extra QC and protocol validation. The biggest variable is data completeness—missing serial/build info is the #1 cause of delays.
Plan the go-live around clinical load. Many sites target late afternoons or weekends, bundling activation with other changes like preset standardization or minor software updates. Build a buffer for training: a 20-minute huddle after activation helps technologists adopt new menus and prevents “feature enabled but unused” scenarios that erode ROI.
F) PAA — “How do I check license status?”
On most platforms, navigate to System → Options/License to view installed features, status, and expiration (if subscription-based). Export the license report as PDF or XML and store it with the activation log. For networked modalities, your asset management or PACS/RIS notes should reflect the change so QA and IT know which rooms support which capabilities.
After service events—HDD swaps, OS reloads, mainboard replacements—re-check status immediately. Keep a simple “tripwire” checklist for front-line techs: if a preset or button disappears, open the license screen first. This quick habit caught a lost Color Doppler option at a cardiac clinic within minutes, avoiding a day of cancelled studies and ensuring a fast re-issue under the provider’s replacement policy.
Pricing & Value: What Drives Cost?
Overview
License pricing reflects the modality, option tier, and where you buy (OEM vs trusted specialist), plus the speed of delivery and level of bundled support. Understanding these drivers helps you compare apples to apples, forecast ROI, and avoid overpaying. This section breaks down real-world cost influences, the economics of downtime, and a simple model to decide whether to license an option or buy new hardware.
A) Cost drivers: modality & option tier
Different modalities carry different price bands because their algorithms and validation footprints vary. Ultrasound options (e.g., CW/Color Doppler, 4D/HDlive, elastography) are usually in the low-to-mid four figures. CT/MRI options (e.g., perfusion, cardiac, advanced recon) trend five figures, reflecting higher R&D and QC burdens. Within each modality, premium tiers—cardiac, perfusion, dual-energy—cost more than general imaging unlocks.
Feature scope also affects pricing. A narrow unlock (e.g., CW Doppler on an echo console) may be cheaper than a suite that adds presets, post-processing, and reporting. A women’s health center chose HDlive only at first to hit budget, then added STIC later when volumes grew. Splitting purchases can smooth cash flow while still capturing demand-led revenue—especially useful for clinics scaling services in stages.
B) OEM vs trusted provider: pricing trade-offs
Buying via OEM generally maximizes warranty alignment and one-vendor governance, but you’ll often see higher list prices and longer scheduling. Hospitals with strict policies accept the premium for unified documentation and centralized support. In one cardiac program, the OEM route added weeks but simplified audit prep because procurement, license issuance, and training all lived in the manufacturer’s ecosystem.
A verified specialist can reduce cost and time-to-value. Many organizations report 30–60% savings and same-day or next-day activation when identifiers are complete. The key is due diligence: insist on device-locked, encrypted keys, a refund/replace policy for incompatibility, and activation logs for audits. One OB/GYN chain enabled HDlive + STIC network-wide over a weekend; the specialist’s documentation satisfied the compliance team and freed budget for probe refreshes.
C) Turnaround time & the economics of downtime
Price alone misses the hidden cost of downtime. If your scanner generates $300–$500 margin per exam and runs 8–12 cases daily, every day lost is $2,400–$6,000 in foregone contribution. Faster activation—especially on ultrasound—often pays for itself within days, which can justify a slightly higher quote that delivers immediate go-live versus waiting weeks for scheduling.
Consider a stroke service launching CT perfusion. Waiting three weeks to enable a reimbursable protocol could delay 20–40 cases, depending on volume. A site that chose quicker activation recovered the license cost sooner simply by avoiding idle capacity. Build your comparison on total time to revenue: quote price + activation lag + QC/training window, not sticker price alone.
D) Bundled support, warranty & total cost of ownership (TCO)
Look beyond the key: ask what’s bundled. Valuable inclusions are remote activation support, replacement keys after board/HDD swaps, minor software updates, and post-activation QC assistance. These reduce friction during future service events, when lost options or version drift can otherwise trigger surprise bills and schedule chaos.
Warranty and audit posture also carry value. A provider that supplies checksums/signatures, activation reports, and option lists simplifies ISO or accreditation reviews. One radiology network standardized on a vendor that kept a secure map of each scanner’s build history. When an OS upgrade was required, the vendor re-issued compatible keys within hours—avoiding re-procurement and keeping rooms on-line.
E) ROI: license vs buying new hardware
A simple model clarifies ROI. Suppose HDlive adds a $60 premium per OB scan and you perform 20 scans/week: that’s $1,200/week. If the license costs ~$3,000, break-even is about 2.5 weeks. After that, it’s margin. This is why clinics often phase options—unlock the highest-yield feature first, then reinvest cash flow into additional packages.
For CT perfusion, assume a $20,000 license and $250 incremental margin per case at 20 cases/month. Monthly contribution is $5,000, so break-even is ~4 months. New hardware rarely competes with that timeline once you account for siting, physics acceptance, and training. Use this framework to compare any option: license cost ÷ (incremental margin × expected volume).
F) PAA — “How much does a medical device license cost?”
Directional ranges (will vary by OEM, region, and software build): Ultrasound options often land in the low-to-mid four figures per feature; CT and MRI packages typically run low five figures, with complex cardiac/perfusion suites higher. C-arm/angio options like DSA/Roadmap/3D rotational tend toward mid five figures. Always request an itemized quote listing option names, build compatibility, delivery time, and support terms.
To get accurate pricing, submit a clean license report, serial/build IDs, and your intended clinical use. Ask for alternatives—single option vs bundle—and for clarity on subscription vs perpetual terms. The best quote enables your specific workflow quickly, preserves compliance and warranty, and includes the support you’ll need when your system inevitably changes over its service life.
G) Exclusive WhatsApp Pricing (Competitive vs Market & OEM)
Readers who contact us on WhatsApp receive special pricing tailored to their modality and option mix—often more competitive than market rates and OEM list pricing. Because we validate your serial/build and intended workflow up front, quotes are precise, activation-ready, and structured to maximize ROI (e.g., phased option bundles, multi-room discounts, replacement-key coverage after board/HDD swaps).
Rapid responses on WhatsApp also shorten time-to-value. Many clinics move from quote to activation the same day when identifiers are complete, capturing revenue sooner and avoiding costly downtime. We provide audit-ready documentation (invoice, activation log, license report) with every job, so your compliance, biomedical, and finance teams have a clean trail for QA and budgeting.
Contact for a WhatsApp-Only Quote
WhatsApp Direct: +967775455559
Click-to-Chat: Chat with us on WhatsApp

Compliance, Warranty & Cybersecurity
Overview
Licensing should improve care without introducing legal, warranty, or security risk. This section explains how to stay compliant with OEM agreements and regulations, keep warranties intact, and protect systems from malware or tampering during activation. You’ll learn what documents to retain, how authorized keys differ from unsafe “keygens,” and how to run remote activations that satisfy internal audits and biomedical QA.
A) Lawful licensing & traceability (audit-ready documentation)
Licenses must be acquired and applied in a way that respects the OEM EULA and local law. Build a clear paper trail: purchase order, device identifiers (model, serial, software/build), pre-activation license report, the issued key’s filename and checksum, activation logs/screenshots, and a post-activation license report. Store these with your asset record so compliance, HTM, and finance can verify “who changed what, when, and why.”
Traceability pays off during accreditation or security reviews. One women’s health center passed a documentation spot-check because their folder held the invoice, device-locked key hash, and before/after license reports—exactly what the auditor asked for. Treat each activation as a formal change: raise a ticket, record downtime window, capture evidence, and close with QC notes. This routine keeps audits short and painless.
B) Warranty alignment & authorized keys
Warranty posture hinges on whether the key is authorized, device-locked, and option-appropriate for your model/firmware. Keys that mirror the OEM’s own mechanism (no bypasses, no patched binaries) generally preserve warranty and service contracts. Ask vendors to confirm in writing that the option is approved for your software build and to provide activation logs suitable for OEM service teams.
Unauthorized methods—generic keygens, modified executables, disabling protections—risk voiding warranty and corrupting software. A cardiac lab once accepted a “too-cheap” Doppler unlock; the next OEM update failed, triggering days of downtime and a paid rebuild. Contrast that with an echo service that used documented, device-locked keys; after a mainboard swap, the provider re-issued a replacement key under terms, and the OEM completed service without dispute.
C) Cybersecurity & secure delivery (data integrity, encryption)
Treat license delivery like any protected software update. Require secure channels (TLS download portal or encrypted email), a checksum/signature to verify integrity, and instructions that do not involve disabling antivirus or Windows protections. Prefer offline import when possible, or controlled, audited remote sessions. Store keys in a restricted repository with least-privilege access.
Common attack paths are removable media and “free” keygens that hide malware. An imaging center avoided a major incident by rejecting a USB-delivered file with no hash and insisting on a signed download instead. After activation, run your standard hardening: verify OS patches, confirm endpoint protection is active, and export fresh license reports. Quick QC scans ensure no unintended changes to image chains or presets.
D) PAA — “Will a medical device license void my warranty?”
Not if it’s authorized, device-specific, and within the OEM’s intended option set for your build. The red flags are bypass tools, generic keys, or features not supported on your firmware/hardware. To stay safe, ask three questions: Is this option listed for my model/build? Is the key device-locked with documentation? Will I receive logs I can show the OEM or an auditor?
Document cooperation between your vendor and service organization. If the OEM performs future maintenance, you want them to see standard option entries and intact system files. Keep a one-page summary in the asset record: date, option names, person performing activation, and links to evidence. This lowers friction during warranty calls and protects uptime.
E) PAA — “Is remote activation safe?”
Yes—when executed with secure tooling and change control. Use vetted screen-share or remote-assist channels, restrict access to the modality workstation only, and schedule a short maintenance window on UPS power. Back up presets, export the current license state, and have a rollback plan. The provider should guide steps in the system’s license manager and remain online through reboot and verification.
A practical pattern: a 30-minute call to collect identifiers, a signed delivery with checksum, then a 60–90-minute activation window. Post-activation, the technologist runs a focused QC (e.g., Doppler angle check, reconstruction kernel test), while HTM attaches the new license report to the CMMS ticket. This workflow has allowed many clinics to go live the same day without compromising security or compliance.
Benefits of Licensed Activation (Authorized Keys)
Overview
Licensed activation turns installed hardware into a higher-capability platform without the cost, delay, or risk of replacement. When keys are authorized, device-locked, and properly documented, you gain stronger compliance posture, warranty continuity, faster time-to-value, and consistent workflows across rooms. This section explains the tangible advantages—financial, operational, and security—so you can justify the decision to leadership and implement it with confidence.
A) Compliance & Audit Confidence
Authorized, device-specific keys create a clean change-management trail: PO, serial/build IDs, pre/post license reports, activation logs, and checksums. This evidence satisfies internal QA and external accreditation because it proves who changed what, when, and why. Aligning options with the OEM’s intended-use matrix also avoids scope creep that can trigger findings during policy or regulatory reviews.
A regional OB network passed a surprise documentation check in minutes by opening a single folder: invoice, key hash, screenshots of the license manager before/after, and a signed activation note. Because options matched the scanner’s cleared indications and software build, the audit ended without remedial actions. That level of traceability is difficult to achieve after informal or unsupported unlocks.
B) Warranty & Service Continuity
Using authorized mechanisms preserves cooperative relationships with OEM service and third-party maintainers. Standard entries appear in the options menu, software files remain intact, and future updates install cleanly. If hardware changes (e.g., mainboard or HDD), reputable providers re-issue replacement keys tied to the new identifiers, keeping your room productive and your warranty posture intact.
Contrast that with generic keygens or patched binaries: updates may fail, logs look suspicious, and you risk a warranty dispute. A cardiac lab that previously suffered a multi-day outage after a bad unlock switched to device-locked, documented keys; when the console later required an OEM patch, the upgrade proceeded normally and the provider supplied a refreshed key within hours—no cancellations, no finger-pointing.
C) Cost Efficiency & ROI vs. New Hardware
Licensing targets software capabilities already supported by your platform, so you avoid capital spend, siting, physics acceptance, and long procurement cycles. The economics are straightforward: small upfront cost, quick go-live, and immediate capture of reimbursable services. Phasing options lets you match investment to demand (e.g., start with Doppler or HDlive, then add elastography or STIC as volumes grow).
Example: at $60 incremental margin per OB case and 20 scans/week, an HDlive license near $3,000 breaks even in ~2–3 weeks. For CT perfusion at $20,000 and $250 incremental margin over 20 cases/month, payback is roughly four months. Few hardware replacements can match that timeline once you include installation, training, and acceptance testing.
D) Uptime & Speed-to-Value
Because keys are delivered digitally and installed via the license manager, activation windows are measured in hours, not weeks. Faster go-live reduces the hidden cost of idle capacity and keeps patient pathways—like stroke or cardio workups—running on schedule. The short maintenance window also minimizes operational risk compared with major upgrades that touch multiple subsystems.
A CT site that enabled perfusion over a weekend started scanning Monday morning with validated protocols. Using the same logic, echo rooms commonly license CW Doppler in a single afternoon. At typical contribution margins ($300–$500 per case, 8–12 cases/day), avoiding just one lost day can protect $2,400–$6,000 in revenue—often more than the premium for faster delivery.
E) Fleet Standardization & Training Efficiency
Licenses help multi-room departments standardize feature sets and presets, so technologists move between rooms without relearning different menus. Consistency improves handoffs, reduces exam variability, and streamlines protocol governance. It also simplifies scheduling: any patient booked for a Doppler or 4D exam can be assigned to any standardized room, which increases utilization.
One network enabled the same cardiac package across three MRI bays and mirrored ultrasound Doppler options across echo rooms. Orientation time for float technologists dropped noticeably, and QA variation on routine measures tightened. Standardization also eases documentation—one set of protocols, one training checklist, and one playbook for QC after software updates.
F) Cybersecurity & Data Integrity
Authorized keys are encrypted and device-locked, delivered through signed portals or encrypted email with checksum verification. This protects against tampering and malware, and it avoids risky behaviors like disabling antivirus or installing unknown executables. Keeping integrity controls intact means fewer surprises during security reviews and smoother collaboration with hospital IT.
A biomedical team rejected a USB-delivered file that lacked a hash and requested a signed download instead. Post-activation, they re-enabled endpoint protections, exported a fresh license report, and ran a brief QC scan—no anomalies detected. By treating license delivery like a controlled software update, they maintained a strong security posture while still going live the same day.
Note: For fast, audit-ready activation and WhatsApp-only pricing, contact us:
WhatsApp Direct: +967775455559 · Click-to-Chat: Chat with us on WhatsApp
FAQs (People Also Ask) to medical device license
1) What is a medical device license key?
A device license key is a machine-locked, encrypted code that unlocks approved software options already built into your ultrasound, CT, or MRI. It doesn’t change hardware; it enables features (e.g., HDlive, CW/Color Doppler, perfusion) via the system’s license manager. You receive documentation and can verify the change in the Options/License screen.
2) Is it legal to buy medical device license keys online?
Yes—provided the key is authorized, device-specific, and enables options already cleared for your model/firmware in your market. Avoid cracks or generic keygens; they violate IP, break cybersecurity controls, and can void warranties. Ask for a paper trail: PO, serial/build, pre/post license reports, and a checksum or signature for the delivered file.
3) Will a license key void my warranty?
Not when activated through authorized mechanisms that mirror the OEM’s intended workflows. Keys should be device-locked and compatible with your build. Problems arise with bypass tools or unsupported features. Keep activation logs and license reports so OEM or service partners can see standard entries and intact system files during future maintenance.
4) How long does activation take?
With complete identifiers (serial, software/build, license report), many ultrasound options go live the same day in a 1–3 hour window. CT/MRI post-processing may require extra QC but still fits a short maintenance slot. OEM scheduling or contract addenda can extend timelines to weeks. The #1 delay is incomplete or inaccurate system info.
5) How do I check which options are active?
On most systems, go to System → Options/License to view installed features and status. Export a license report (PDF/XML) before and after activation. If a preset disappears after service (e.g., HDD swap), check this screen first—fast detection helps vendors re-issue replacement keys tied to new identifiers without disrupting the schedule.
6) Can refurbished or used systems be licensed?
Yes. Refurbished consoles are commonly upgraded by enabling options that match their model and firmware. Best practice is to export a clean license report, confirm probe compatibility, and plan a short QC session after activation. Many refurbishers bundle licenses to deliver “feature-complete” systems that pass incoming QA and hospital audits.
7) What information do you need to generate a key?
Model and serial, exact software/build (e.g., GE BTxx, Philips EPIQ x.x.x), the license/options report, probe list, and any recent hardware changes. Screenshots or a short screen recording of the service/about pages help prevent typos. These identifiers are the fingerprint that produces a device-locked, encrypted key matched to your system.
8) Are medical device license transferable between machines?
No. Legitimate keys are device-locked to your serial/build and won’t load on other units. If a mainboard or storage device is replaced, reputable providers can re-issue a replacement key against the new identifiers under documented terms. This preserves uptime and avoids compliance issues with unauthorized file reuse.
9) What happens after firmware or OS updates?
Authorized keys survive routine, supported updates. Before major upgrades, confirm compatibility with your vendor and export a pre-update license report. After the update, verify features in the options menu and run focused QC (e.g., Doppler angles, reconstruction kernels). If identifiers changed, request a refreshed key using the new build details.
10) How much do medical device licenses cost?
Directional ranges vary by modality and option tier. Ultrasound features are often low-to-mid four figures; CT/MRI post-processing tends to low five figures, with cardiac/perfusion or dual-energy higher. Compare on total value: delivery speed, documentation quality, and replacement-key coverage—these can outweigh small price differences.
11) Is remote activation secure?
Yes—when delivered via encrypted channels with checksum/signature verification and executed in a controlled maintenance window. Do not disable antivirus or install unknown executables. Use the system’s license manager, maintain a change log, and store the signed files in restricted folders. Treat it like a controlled software update with post-activation QC.
12) What’s the ROI versus buying new hardware?
Licensing targets software capabilities, so you avoid capital, siting, and long procurement. Example: a ~$3,000 HDlive option with a ~$60 uplift on 20 OB scans/week breaks even in ~2–3 weeks. A ~$20,000 CT perfusion option at ~$250 incremental margin and 20 cases/month pays back in ~4 months—far faster than hardware replacement.
Conclusion to medical device license
Overview
Licensed activation is the fastest, lowest-risk way to expand capability on equipment you already own. By distinguishing software option keys from regulatory approvals, you can add revenue-driving features while preserving compliance, warranty posture, and cybersecurity. With a clean paper trail, device-locked keys, and a brief maintenance window, most sites realize value within days—not months—while standardizing workflows across rooms and reducing training friction.
A) Key takeaways
Authorized, device-specific keys transform base configurations into clinically capable systems without capital replacement. Matching options to your model/firmware and documenting each step—PO, serial/build IDs, pre/post license reports, checksums—creates an audit-ready trail that satisfies QA and accreditation. This is the difference between sustainable scaling and risky shortcuts that jeopardize warranty, updates, or future service events.
Equally important is the conceptual clarity: a software license enables approved features; it does not substitute for regulatory market approval. Keep indications and intended use aligned with OEM documentation, and ensure your policies reflect that separation. Teams that internalize this distinction avoid scope creep, pass audits faster, and collaborate smoothly with both OEM service and hospital IT.
B) Decision framework (license vs new hardware; OEM vs specialist)
Choose a license when the bottleneck is features, not throughput. If the desired capability exists for your platform and patient volume won’t exceed current hardware limits, a software key wins on time and ROI. Opt for hardware only when you need fundamental performance leaps—detector, gradients, slice count—or when your platform is out of support and can’t host the option safely.
For sourcing, OEM simplifies single-vendor governance but often adds time and list price. A verified specialist typically delivers in hours with 30–60% savings when identifiers are complete. Mitigate risk by requiring device-locked encryption, compatibility checks, refund/replace terms, and activation logs. A women’s health network used this framework to green-light HDlive now and STIC later, matching spend to demand.
C) Implementation checklist (pre, during, post)
Pre-activation: Export serial/build, license report, probe list, and current presets. Validate option–build compatibility and plan a UPS-backed maintenance window. Share a brief plan with radiology/OB leadership and HTM so everyone knows when rooms are offline and what to test afterward. A five-minute intake checklist prevents the most common delay: incomplete identifiers.
During & post: Install through the license manager, reboot if required, and immediately verify options in the features menu. Run focused QC—Doppler angles and frame rates on ultrasound; reconstruction kernels or perfusion maps on CT; gated sequences on MRI. Export a fresh license report, attach evidence to your CMMS ticket, and update protocols so technologists see new presets at the next login.
D) KPIs & ongoing optimization
Track time-to-activation, uptime, case volume, and incremental margin per exam. For example, a $60 uplift on 20 OB scans/week adds ~$1,200 weekly; break-even on a ~$3,000 option lands in 2–3 weeks. For CT perfusion, a $20,000 license with $250 incremental margin at 20 cases/month pays back in ~4 months. Add QA metrics—reject rates, repeat scans, and report turnaround—to capture qualitative gains.
Standardization yields durable benefits. Monitor cross-room protocol parity, training minutes for float techs, and variance in key measures (e.g., Doppler VTI). A site that mirrored CW Doppler across echo rooms saw onboarding time drop and fewer rescheduled studies. Review these KPIs quarterly; if demand justifies it, phase in the next option (e.g., elastography after HDlive) to compound returns.
E) Get help — fast, medical device license OEM-compliant activation & WhatsApp-only pricing
If you’re ready to proceed, we’ll validate identifiers, confirm compatibility, and deliver encrypted, device-locked keys with audit-ready documentation. Most ultrasound activations complete in a single afternoon; CT/MRI post-processing options follow with structured QC. We also support replacement keys after board/HDD swaps, minimizing downtime during future service events.
For a special WhatsApp quote—often more competitive than market and OEM list—reach out now. You’ll receive a precise, activation-ready proposal matched to your workflow, with refund/replace terms and remote guidance included.
WhatsApp Direct: +967775455559
Click-to-Chat: Chat with us on WhatsApp
Also read about information on Buy CT Scan License And Software | Secure Activation
, click here. (https://bmeser.com/ct-scan-license-and-software/)
Here’s a clean add-on block you can paste after the conclusion:
Choosing the right medical device license helps clinics unlock approved features on installed systems without replacing hardware. When you buy a medical device license from an authorized source, you receive a device-locked medical device license key, audit-ready documentation, and OEM-compliant activation. This keeps your medical device license aligned with warranty terms while enabling options that match your model and software build.
For ultrasound, a medical device license can enable HDlive, STIC, or CW/Color Doppler; for CT/MRI, a medical device license may add perfusion, cardiac, or advanced reconstruction. Each medical device license is generated from your serial and build, so compatibility and encryption are verified. Hospitals standardize rooms by applying the same medical device license across sites to reduce variability and speed training.
Before purchase, export identifiers and a license report, then confirm the medical device license supports your indication in your market. During activation, load the medical device license through the system’s license manager, reboot if required, and verify features. The right medical device license accelerates ROI: faster go-live, less downtime, and clear logs for audits—all without capital replacement.
For help choosing or to buy a medical device license today, contact us on WhatsApp +967775455559 or start a chat: https://wa.me/message/NRDFVKNYDTUNM1.





Bosphorus sunset cruise
August 31, 2025Bosphorus sunset cruise The lunch stop was delicious and well-timed.
ebrahim
September 7, 2025what?